Hyper Cross-linked, Based on non-animal Ha, Residual-free,
Improved Density and elasticity Technology
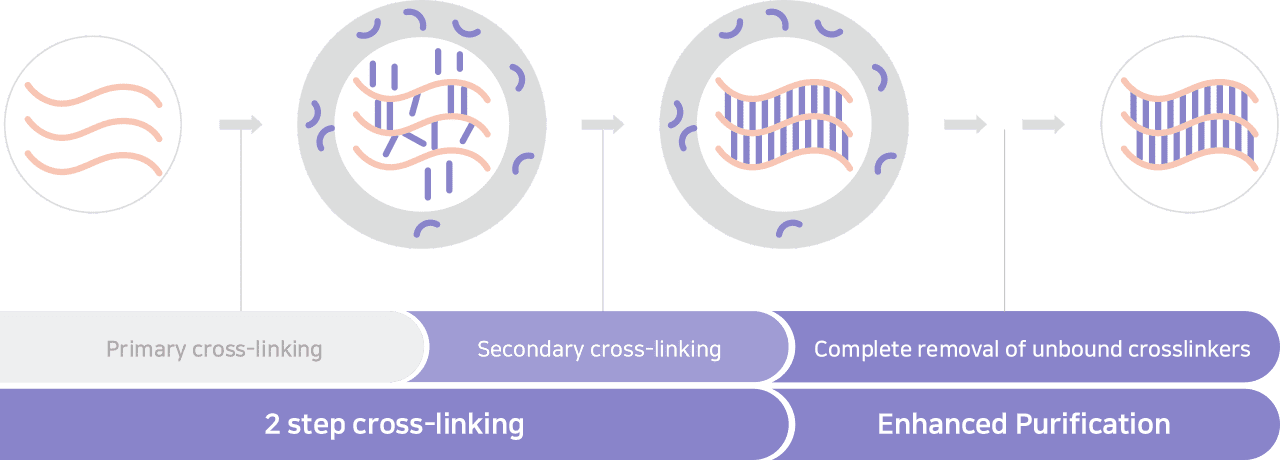
The unique manufacturing process of exquiller, characterized by an ambivalent monophasic
and biphasic HA driven via an enhanced crosslinking technology and optimized purification process,
helps guaranteeing the safety, the long-lasting effect and high satisfaction rate.
Enhanced safety
exquiller passed 1st, 2nd, 3rd clinical trials of Korean Institutional Review Board, and its safety was officially approved by Korean government and EU (CE) (*Not tested on animals)
Long-lasting effect
exquiller ambivalence and high cross-linking rate ensure a long-lasting effect.

Hign satisfaction
Approved clinical data and majority of reports from patients show(prove) long lasting molding results of exquiller..
Raw material stability
Using selected raw material ( HA) Which has received CE certification and been found very safe
Advanced cross-linking
Via enhanced ambivalent cross-linking through HYBRID technology,
BDDE residue is highly minimized to higher standards.
Post Sterilization
Through the Post sterilization process, the risk of microbial contamination has been minimized.
Performance Test
| Standard | Result |
|
|---|---|---|
| Appearance | No impurities, transparent and colorless gel | Pass |
| HA Concentration | 21.6 ~ 26.4 mg | 24.1 mg |
| pH | 6.5 ~7.5 | 7.10 |
| Residual BDDE | < 2 ppm | Not Detected |
| Endotoxin | < 20 EU | < 0.100 EU |
| Volume | > 1.0 mL | Pass |
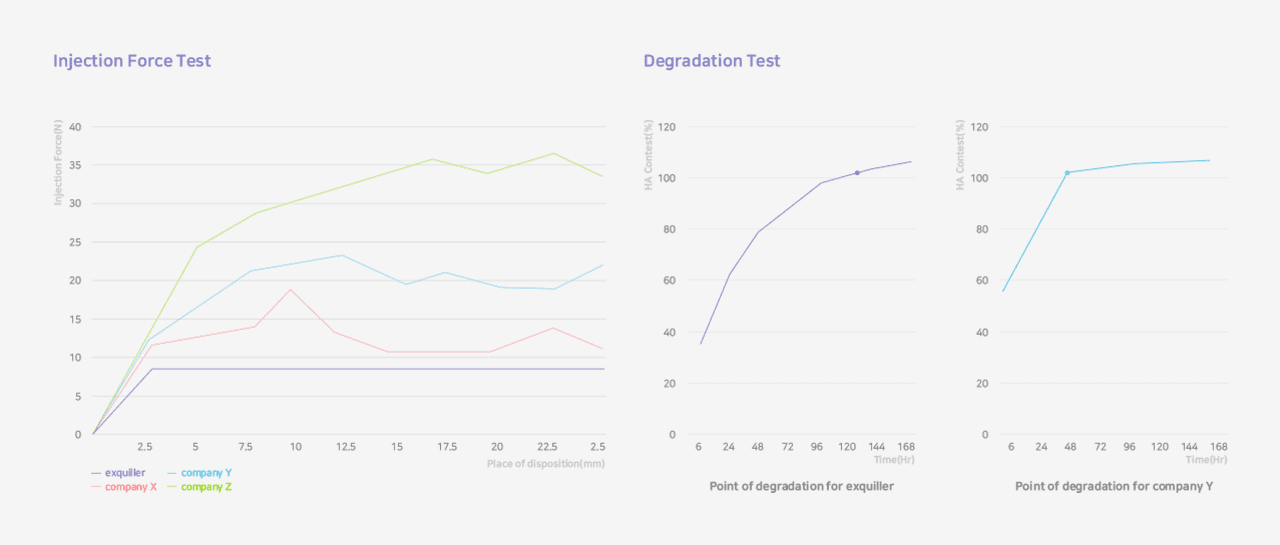
Low And Steady Injection Force
Long Lasting Natural Molding Effect
exquiller deep hugs the shape very
easily while giving the most natural volume
formation